Viridian Therapeutics announced on Tuesday that its experimental drug, veligrotug, has demonstrated significant efficacy in reducing symptoms of thyroid eye disease (TED) in a late-stage clinical trial. This development positions Viridian’s treatment as a potential competitor to Amgen’s widely used therapy, Tepezza.
The promising trial results led to a 14 percent increase in Viridian’s share price, which reached $16.19 during morning trading. The drug successfully met both primary and secondary endpoints in patients suffering from TED, a rare condition affecting an estimated 90 to 300 individuals per 100,000 in the U.S.
Jefferies analyst Michael Yee highlighted the potential advantages of veligrotug, noting that its less frequent dosing regimen compared to Tepezza could offer a more convenient alternative. “Today’s data is very encouraging, with the potential for a shorter-duration and more convenient treatment option, along with a favorable side effect profile,” Yee remarked in an email.
Yee projects that veligrotug could achieve peak sales of up to $2 billion as a TED treatment. In comparison, Tepezza generated $479 million in sales for the quarter ending June 30. Amgen acquired Tepezza through its $277.8 billion acquisition of Horizon Therapeutics.
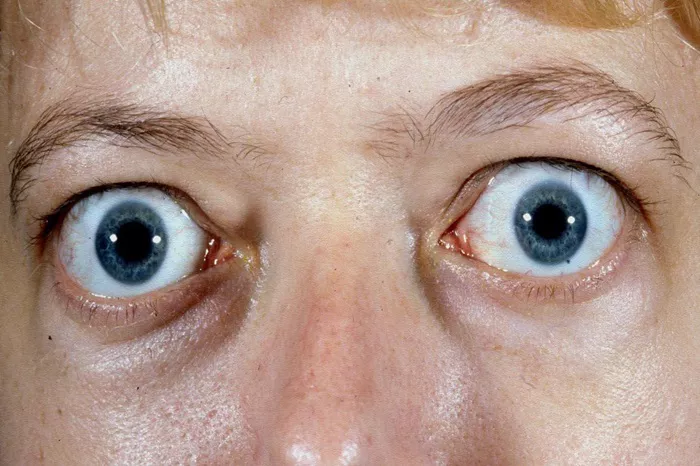
TED, which often occurs in patients with Graves’ disease—a condition causing an overproduction of thyroid hormones—leads to inflammation and damage of the tissues surrounding the eyes. According to Viridian, veligrotug achieved a reduction of at least 2 millimeters in eye bulging in 64 percent of patients, after adjusting for placebo effects, following 15 weeks of treatment.
The company also reported that 5.5 percent of participants in the 113-patient study experienced treatment-related hearing impairment, a rate lower than anticipated. Truist analyst Srikripa Devarakonda acknowledged the drug’s effectiveness but noted that the hearing loss side effect might leave room for future competitors.
Viridian plans to release data from a second late-stage study towards the end of this year and aims to seek regulatory approval in the second half of 2025.
Related topic:
How To Reduce Blue Veins Under Eyes?
How To Get Rid Of Blue Eye Quickly?