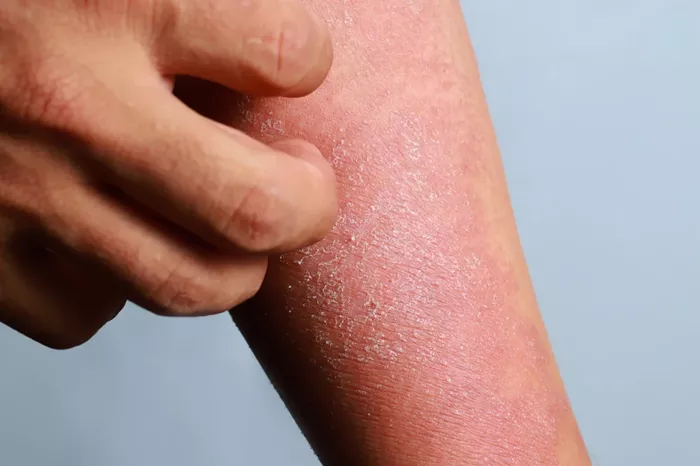
The U.S. Food and Drug Administration (FDA) has approved Ebglyss (lebrikizumab-lbkz), a new biologic treatment for moderate-to-severe atopic dermatitis, offering a much-needed option for patients who do not respond adequately to topical treatments like steroid creams. This injectable medication is approved for adults and children aged 12 and older and is administered once a month.
Ebglyss belongs to a class of drugs known as targeted interleukin-13 (IL-13) inhibitors, which aim to reduce inflammation associated with eczema. Dr. Amy McMichael, a dermatologist at Atrium Health Wake Forest Baptist, noted the strong data supporting the drug’s efficacy, particularly from studies involving diverse skin tones, indicating its consistent effectiveness across different demographics.
In clinical trials, approximately 38% of participants achieved clear or almost-clear skin within 16 weeks of treatment, compared to just 12% with placebo. Moreover, 43% reported significant itch relief by the same time frame. Side effects included eye inflammation and injection site reactions, with a risk of shingles.
Notably, Ebglyss also demonstrated effectiveness in a small trial specifically involving people of color, a group often underrepresented in clinical research. This study highlighted similar results to broader trials, showing the treatment’s promise in alleviating severe symptoms of atopic dermatitis in diverse populations.
The list price for Ebglyss is set at $3,500 per pen, though out-of-pocket costs may vary based on insurance coverage. Eli Lilly, the drug’s manufacturer, is working to ensure patient access through support programs and collaborations with health insurers. Ebglyss is expected to be available in the U.S. in the coming weeks.
Related topic:
CurrentBody RF Device: Salon Results at Home
SumaNurica’s Anti-Wrinkle Eye Serum Shines at 2024 Emmy Awards