Avita Medical (ASX: AVH) has announced that its RECELL GO mini disposable cartridge has received approval from the U.S. Food and Drug Administration (FDA), marking a significant expansion for the company’s pioneering skin regeneration platform.
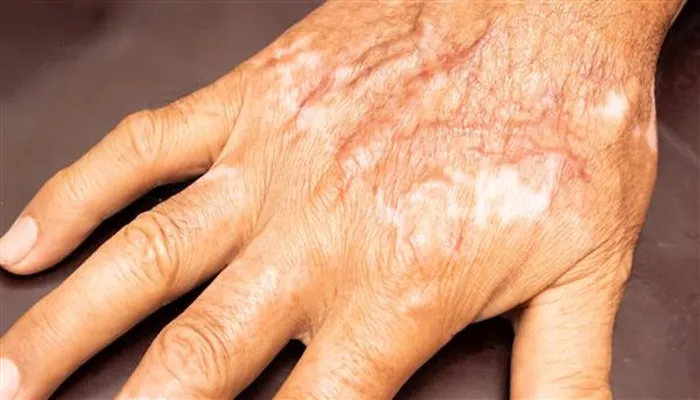
The RECELL system, originally approved by the FDA in 2018 for severe burn treatment, has seen successive approvals over the years. In 2022, the platform was authorized for use in repigmentation of vitiligo lesions, and in 2023, it gained approval for the treatment of full-thickness skin defects, such as injuries that expose bone, fat, or muscle.
RECELL works by harvesting a small sample of the patient’s own skin — just one square centimeter — which is then transformed into “Spray-On Skin” capable of treating up to 80 square centimeters of damaged tissue. The Spray-On Skin is applied directly to the wound, stimulating healing not only from the edges of the wound but also from within, accelerating recovery times. While typical wound healing takes 3-6 weeks, RECELL-treated wounds can heal in as little as 1-2 weeks.
The RECELL GO mini is an extension of this innovative platform, designed specifically for smaller wounds. The new device features disposable cartridges, in contrast to the original RECELL system’s single-use components. RECELL GO mini can treat wounds up to 480 square centimeters, while the standard RECELL GO device handles larger wounds up to 1,920 square centimeters, or roughly three square feet. By using the same processing device as the original system but with a smaller cartridge, the mini version minimizes waste and offers a more flexible, cost-effective solution.
Jim Corbett, CEO of Avita Medical, highlighted the significance of this FDA approval, stating, “The FDA approval of RECELL GO mini strengthens our ability to provide clinicians with fit-for-purpose solutions that meet the diverse needs of patients with full-thickness wounds.” He added that the device’s smaller size could drive broader adoption of the RECELL platform in clinical settings.
Avita plans to launch the RECELL GO mini in trauma and burn centers in the first quarter of 2025. Following the announcement, shares of Avita Medical saw a 5.25% increase, reaching $4.01.
Related topic:
North India Plastic Surgeons Convene for Conference on Advancements
Sofie Pavitt Brings Acne Skincare Line to Sephora
FDA Approves Xyngari Name for Dermata’s Acne Treatment